To receive a quote for our “Single Pallets, Parcels & Documents” service you are required to create an online cash account.
We understand the strict regulations governing the transportation of pharmaceutical products. Our team of experts is well-versed in the industry’s regulatory requirements, including Good Distribution Practices (GDP), cold chain management, and customs procedures. We ensure strict adherence to these regulations, providing you with peace of mind and minimising the risk of non-compliance.

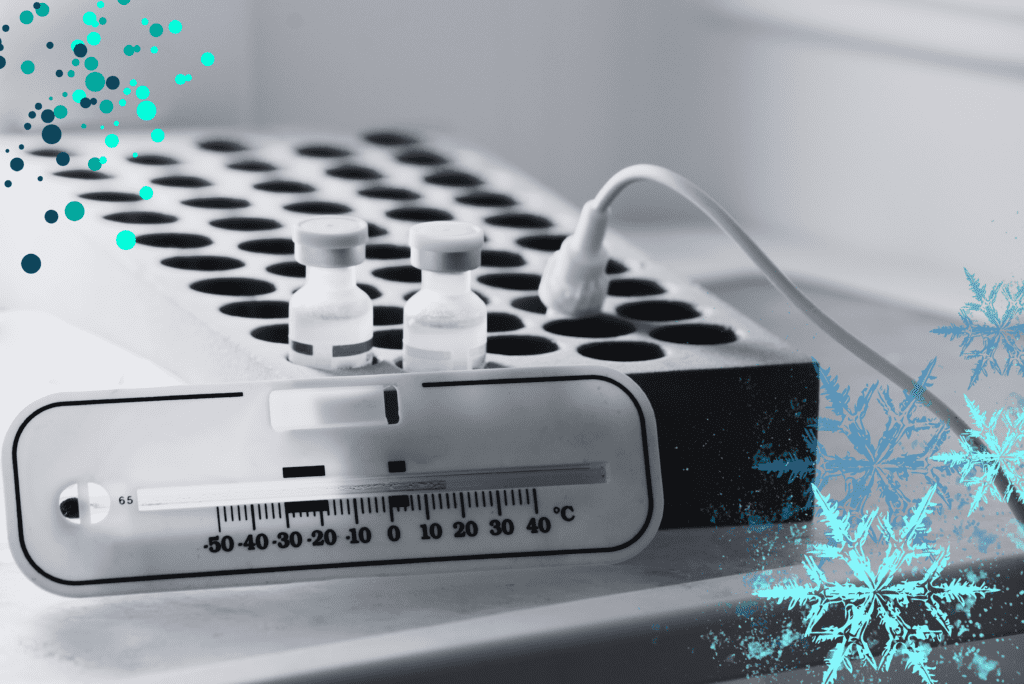
Many pharmaceutical products, such as vaccines, biologics, and sensitive medications, require strict temperature control to maintain their efficacy and stability. We specialise in temperature-controlled logistics, employing state-of-the-art facilities and equipment, such as refrigerated containers (reefers) and temperature monitoring systems, to ensure that your products are transported within the required temperature range.

Our expertise extends to managing the cold chain, which is crucial for preserving the integrity of temperature-sensitive pharmaceuticals throughout the transportation process. We meticulously monitor and maintain the desired temperature conditions, from pickup to delivery, ensuring that your products remain safe and effective.
International shipping of pharmaceutical products involves complex regulatory documentation, including import/export permits, certificates of analysis, and product registrations. Our experienced team manages the entire documentation process, ensuring compliance with international regulations and minimising potential delays at customs.

At WorldSora we recognise the high importance of reliable and secure transportation in the pharmaceutical and medical manufacturing industry. Our bespoke international delivery and logistics solutions are designed to meet the unique needs of this sector, ensuring the safe and efficient transportation of pharmaceutical products and medical supplies across the globe.